Have you ever wondered what secrets are hidden inside a small lithium battery? This article will take you deep into the components of a lithium battery and reveal how they work together to provide powerful power for your device. We will disassemble a lithium battery and analyze key components such as the positive electrode, negative electrode, separator, electrolyte, etc. one by one, so that you can thoroughly understand the internal structure of a lithium battery. Understanding these components can help you better understand the performance and precautions of lithium batteries.
Last updated: May 18, 2025 | Estimated reading time: 8 minutes
Table of Contents
ToggleBasic knowledge of lithium batteries
Lithium-ion batteries are rechargeable batteries that store energy by reversibly inserting and deintercalating lithium ions between the positive and negative electrodes. The lithium ions shuttle between the electrodes, enabling the battery’s charge and discharge process. Lithium batteries are becoming increasingly popular due to their lightweight, high energy density and rechargeable properties, and are widely used in laptops, mobile phones, electric vehicles and other devices.
Detailed disassembly of lithium battery cells
A typical lithium-ion battery cell consists of the following main components:
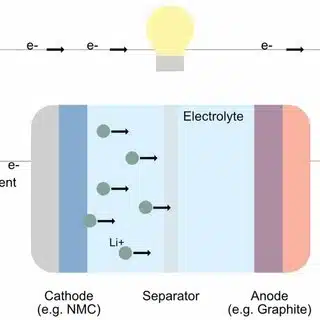
Cathode: The positive electrode is the anode of the battery, where lithium ions and electrons flow in (when charging) and flow out when discharging.
Positive electrode materials are usually composed of metal oxides, such as lithium cobalt oxide (LiCoO2), lithium manganese oxide (LiMn2O4), lithium iron phosphate (LiFePO4), lithium nickel cobalt manganese oxide (LiNiMnCoO2, abbreviated as NMC) and lithium nickel cobalt aluminum oxide (NCA).
These materials have their own advantages and disadvantages, and differ in energy density, thermal stability, cost-effectiveness, etc. For example, lithium cobalt oxide (LiCoO2) is known for its high energy density and is suitable for portable electronics; lithium iron phosphate (LiFePO4) is favored for its excellent thermal stability and safety and is often used in electric vehicles and energy storage systems. NMC has the advantages of energy density, power density and stability.
The positive electrode usually consists of aluminum foil as the current collector, on which the positive electrode active material mixture is coated.
Anode: The negative terminal of the battery is the source of electrons during discharge.
The negative electrode material is usually made of carbon materials, such as graphite, and silicon or a mixture of graphite and silicon is also used.
Graphite is the most commonly used anode material due to its high conductivity, low cost, and stable structure. 7 Silicon anodes offer higher energy density but face challenges in terms of volume expansion and cycle life. Some anode improvement schemes also dope graphite anodes with a small amount of silicon to improve performance characteristics and energy density.
The negative electrode usually consists of copper foil as the current collector, on which the negative electrode active material is coated.
The separator is located between the positive and negative electrodes to prevent them from directly contacting each other and causing a short circuit. The separator is usually a porous membrane made of plastic such as polyethylene (PE) or polypropylene (PP). It allows lithium ions to pass through, allowing the battery to charge and discharge normally.
The electrolyte is the medium through which lithium ions move between the positive electrode and the negative electrode. It is usually a mixture of a lithium salt (such as lithium hexafluorophosphate LiPF6) dissolved in an organic carbonate solvent (such as ethylene carbonate EC, dimethyl carbonate DMC). It has good ionic conductivity, chemical stability and electrochemical stability.
The current collector is used to conduct electrons from the electrode to the external circuit. The positive electrode current collector is usually aluminum foil, while the negative electrode current collector is usually copper foil.
The casing is used to enclose and protect the internal components of the battery. Typically made of aluminum, steel, or plastic, depending on the type of battery (cylindrical, prismatic, or pouch).
Key components for battery pack assembly
In addition to the above-mentioned battery cell components, lithium battery assembly also requires the following key components:
- Battery Management System (BMS): The battery management system is the “brain” of the battery pack, responsible for monitoring the battery status, controlling the battery charging and discharging process, and ensuring battery safety and performance. Learn more about battery pack design.
- Bus bars: They are used to provide a low resistance connection between battery cells and terminals.
- Thermal interface material: It is used between the battery cell and the casing or cooling channel to maximize heat transfer.
- Insulation Materials: Located inside the battery casing, they prevent components from contacting and causing a short circuit. Common insulation materials include polyester film and ceramic coatings to ensure the safety and reliability of the battery during operation.
- Enclosure: It is used to protect all internal components.
Risks and precautions of disassembling lithium batteries
There are certain risks in disassembling lithium batteries, including:
- Fire: Lithium batteries contain flammable electrolyte which may present a fire hazard if mishandled.
- Chemical exposure: Chemical substances in lithium batteries may be harmful to the human body and should be avoided during disassembly.
- Short circuit: During the disassembly process, if the positive and negative poles come into contact, it may cause a short circuit, generate high temperature or even explosion.
- Ensure good ventilation: Perform disassembly in a well-ventilated area to reduce the effects of harmful gases.
- Wear protective equipment: Wear protective gloves, mask and goggles to avoid contact with harmful substances.
- Use insulated tools: Use insulated tools to prevent short circuits.
- Discharge: Discharge the battery as much as possible to reduce the risk.
- Handle with caution: Remove the casing carefully to avoid puncturing the battery cells.
- Separate storage: Separate disassembled components into appropriate containers for recycling or disposal.
- Seek professional help: If you are not sure what to do, please seek help from our Hongyitai professionals.
Conclusion
Lithium batteries are made up of multiple complex components that work together to provide energy for our devices. Understanding the internal structure of lithium batteries and the risks of disassembling them can help us use and handle lithium batteries more safely and efficiently. Through the introduction of this article, I believe you have a deeper understanding of lithium batteries. With the continuous advancement of technology, we look forward to greater breakthroughs in energy density, safety, environmental protection and other aspects of lithium batteries in the future.
