Have you ever wondered what makes your phone last all day? What makes your laptop work even without being plugged in? Behind these devices, there is often a common heart, that is, lithium batteries.
But how does this energy we rely on every day work inside, storing and releasing energy? The goal of this article is to reveal the core secrets of how lithium batteries work in the simplest and most straightforward way.
Table of Contents
ToggleThe core components of lithium batteries
The core components of lithium batteries mainly include the following aspects:
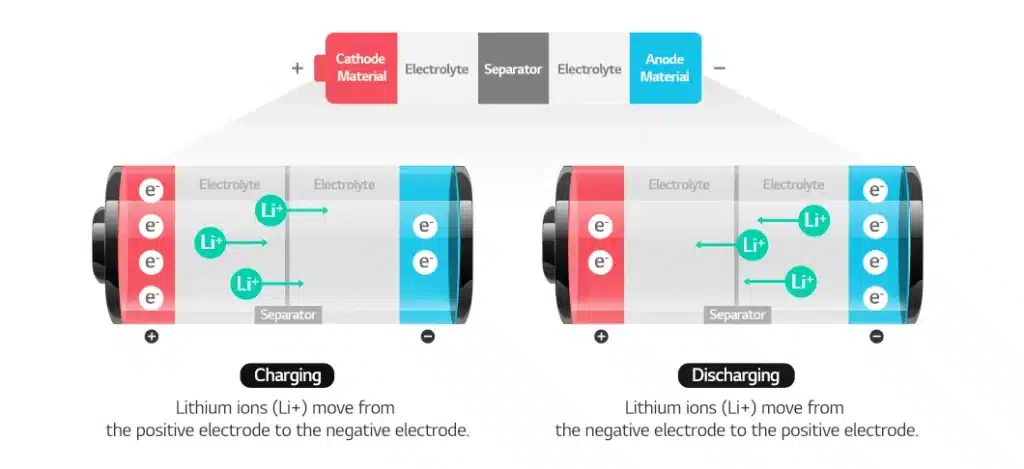
The positive electrode material is the core component of lithium batteries and determines the capacity, voltage and performance of the battery. Commonly used positive electrode materials include lithium cobalt oxide, lithium manganese oxide, lithium iron phosphate and ternary materials (polymers of nickel, cobalt and manganese). The positive electrode material directly affects the overall performance and cost of the battery and usually accounts for a large proportion of the electrode material mass. The current price of lithium cobalt oxide is about 215,000 yuan/ton, and its price increase ratio is high compared with the beginning of the year.
Negative electrode materials generally use natural graphite or artificial graphite as a “storage warehouse” for lithium ions, responsible for the embedding and release of lithium ions. Negative electrode materials play a key role in improving battery capacity and cycle performance.
Electrolyte is the medium for lithium ions to conduct between the positive and negative electrodes, usually composed of organic solvents and lithium salts. It can ensure the efficient migration of lithium ions and is the key to achieving high voltage and high energy density of lithium batteries. It has three types: solid, liquid and colloidal.
The separator is a porous film located between the positive and negative electrodes, usually made of polyethylene and polypropylene. It isolates the positive and negative electrodes and prevents short circuits, while allowing lithium ions to pass through to ensure battery safety and performance.
The protection board is the brain of the lithium battery pack, responsible for real-time monitoring of battery voltage, temperature, current and other parameters, protecting the battery from dangers such as overcharge, over discharge, overheating and short circuit, and ensuring safe and efficient operation of the battery. It has two types: PCM and BMS.
Lithium battery charging and discharging process
The charging and discharging process of lithium batteries mainly involves the embedding and de-embedding of lithium ions between the positive and negative electrodes. The specific process is as follows:
Charging stage
Pre-charging: When the battery voltage is lower than 3V, a small current (about 1/10 of the rated charging current) is used for trickle charging. The purpose is to safely wake up the deeply discharged battery and prevent damage.
Constant current charging: after the battery voltage rises to 3V, the charger charges at a constant higher current (usually 0.2C to 0.5C, C is the unit of battery capacity), and the battery voltage gradually increases until it reaches the set maximum voltage (usually 4.2V/cell).
Constant voltage charging: when the battery voltage reaches 4.2V, the charger switches to constant voltage charging to maintain the voltage stable. The charging current gradually decreases. As the battery is nearly fully charged, the current will drop to a very low level.
Charging is terminated when the charging current drops to a preset level of about 0.1C. For safety reasons, the charger also monitors temperature and charging time to prevent overcharging.
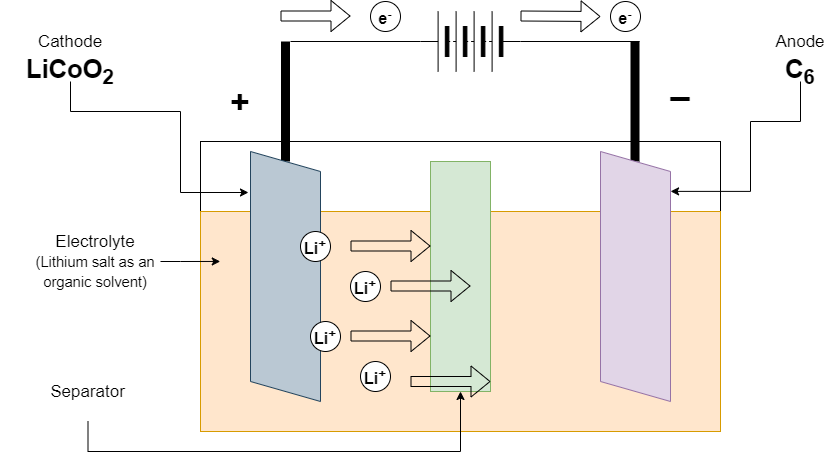
Positive electrode: LiCoO2→Li1−xCoO2+xLi++xe−
Negative electrode: xLi++xe−+6C→LixC6
Discharge phase
During discharge, lithium ions are removed from the negative electrode and migrate to the positive electrode through the electrolyte, and the battery releases electrical energy for use by the external load. The discharge voltage is generally between 2.8V and 4.2V, with a typical nominal voltage of 3.7V. During the discharge process, the number of lithium ions in the negative electrode decreases, while the number of lithium ions in the positive electrode increases.
During charging: Lithium ions are deintercalated from the positive electrode material, migrate through the electrolyte to the negative electrode, and are intercalated into the negative electrode material (such as graphite).
During discharge: lithium ions are deintercalated from the negative electrode, migrate back to the positive electrode through the electrolyte, embed into the positive electrode material, and release electrical energy.
Its main chemical reaction formula is as follows:
The following is the main chemical reaction formula:
Why lithium Ion
You may be curious, why lithium has become the main element of batteries?
First of all, lithium is very light. Its atomic number is 3 and its atomic weight is about 6.94, which is one of the smallest atomic weights of all metal elements.
Second, lithium is chemically very reactive, meaning it easily loses electrons (when discharging) and easily recombines (when charging), making it a natural fit for carrying energy.
Lithium has a high energy density and can store relatively more energy.
Lithium batteries need a safety steward, a battery management system. It monitors the battery status, voltage, current and temperature in real time. Once the BMS detects abnormal data, it will take immediate action to cut off the circuit to protect the battery.
Conclusion
Lithium ions flexibly shuttle between the positive and negative poles inside the battery, while electrons flow in the external circuit, cleverly achieving the storage and release of energy. This is the power that drives portable devices.
