We all notice that after using mobile phones, laptops and other electronic products for 1-2 years, the battery life is not as good as before. So why do these batteries age and their performance deteriorate over time? Battery degradation generally refers to capacity degradation and increased internal resistance, and reduced discharge capacity. The following article will explore in depth the causes of degradation and how to extend battery life.
Table of Contents
ToggleWhy do batteries degrade

Battery degradation is not a superficial phenomenon, but a chemical and physical failure inside lithium ion batteries. The following are the main mechanisms that cause degradation:
SEI film growth
When a lithium battery is charged for the first time, the electrolyte reacts on the surface of the negative electrode to form a thin and dense passivation layer called the solid electrolyte interface (SEI). This layer is crucial and plays a protective role. As the number of charge and discharge increases, this layer will become thicker, hindering the speed of lithium ions entering and exiting, resulting in increased internal resistance. This is the main reason for the degradation of lithium batteries.
Lithium precipitation
Also called lithium dendrites. Under normal conditions, lithium ions should be smoothly embedded into the layered structure of the negative electrode material during charging. However, lithium precipitation means that the lithium ions fail to enter successfully, but are directly deposited on the surface of the negative electrode to become metallic lithium. In this way, lithium ions are wasted and other lithium ions are blocked from embedding. If these spiked dendrites continue to grow, they may pierce the diaphragm, causing an internal short circuit and posing a safety risk.
Loss of active materials and structural damage
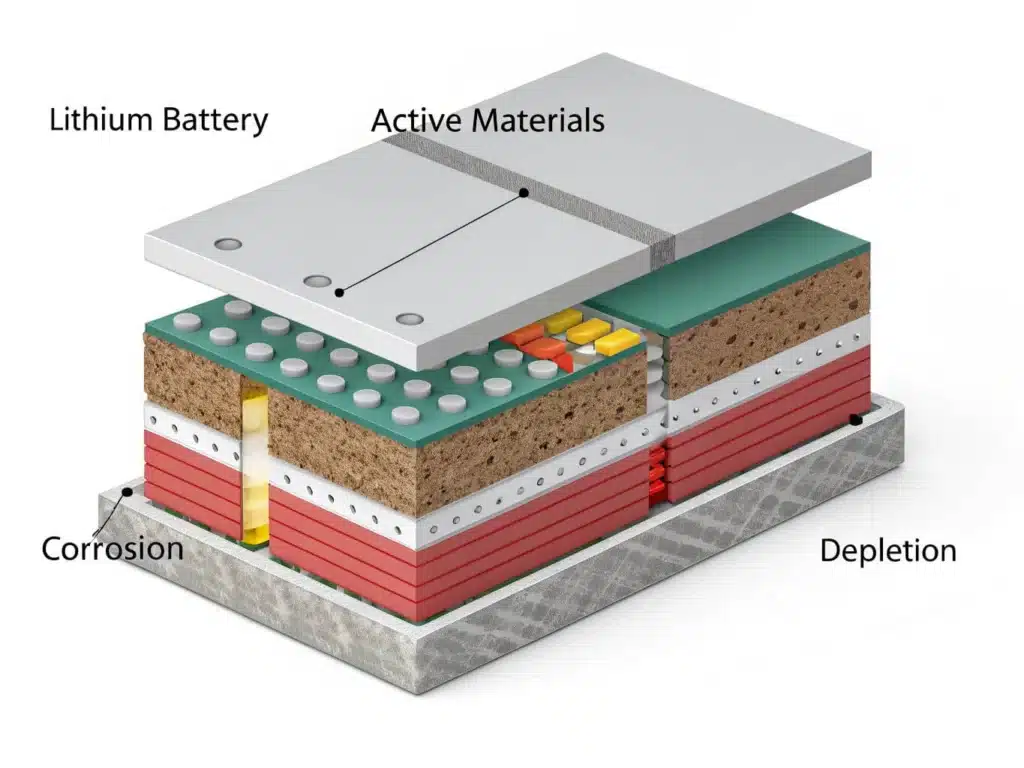
As time goes by, the positive and negative electrode materials inside the lithium battery will crack, break, and even some components will be dissolved into the electrolyte. These structural changes will directly lead to a decrease in the number of active substances that can participate in the reaction, that is, a decrease in available capacity. At the same time, after the material structure is destroyed, the path of lithium ion insertion or extraction changes, thereby increasing internal resistance.
Electrolyte decomposition
The electrolyte is a channel for lithium ions to be free. Under high temperature or high pressure, it will decompose and produce gases such as CO2, CO, C2H4, which can cause battery bulging in severe cases. Because the effective components of the electrolyte decompose, the ion conductivity decreases. The byproducts of its decomposition may adhere to the electrode surface, further hindering the transmission of lithium ions.
Increased internal resistance
The above reasons interact with each other, causing the internal resistance of the battery to increase, which will increase the energy conversion damage of the battery, manifested as battery heating, slower charging speed, and rapid voltage drop.
Extreme temperatures
High temperature environments are extremely detrimental to lithium batteries. According to basic chemical principles, increased temperature significantly accelerates the rate of chemical reactions, and high temperature accelerates almost all major battery internal decay processes.
Although low temperature environments usually do not accelerate chemical reactions as much as high temperature, they do pose significant risks when charging. At low temperatures (especially below 0°C), the negative electrode’s ability to receive lithium ions decreases. If charging is performed at this time, lithium ions will not have time to embed into the negative electrode, and lithium precipitation will easily occur on the negative electrode surface, forming metallic lithium dendrites.
Discharge depth
Running out of battery power or even over-discharging it can also cause irreversible damage. Under certain conditions, over-discharging can cause the copper current collector at the negative electrode to dissolve and deposit, resulting in internal micro-short circuits or performance degradation.
Overcharging
When the battery is charged to 100% for a long time, the electrochemical pressure on the internal electrode materials (especially the positive electrode) increases. Maintaining this high voltage state for a long time will accelerate the growth of the SEI film and the oxidation and decomposition of the electrolyte and other side reactions.
High charge and discharge rate
Large currents will generate more Joule heat, increase the battery temperature, and indirectly accelerate all decay caused by high temperature. Secondly, rapid lithium ion insertion or extraction will cause more damage to the electrode material structure.
Battery aging
Cyclic aging refers to the fact that each charge and discharge is accompanied by the growth of tiny SEI films and the loss of active materials.
Calendar aging is the natural decline in battery performance over time, even if the battery is not used.
Degraded lithium battery results

The aging of lithium batteries is not silent, it will leave many signs in daily use. When your device shows some of the following phenomena, it is likely that the battery is experiencing degradation:
Capacity decreases. This is the most intuitive and easiest way for you to feel the attenuation. A mobile phone that used to be able to be used for a whole day on a full charge may now need to be recharged in the afternoon; the dashboard of an electric car shows that the range after a full charge has been significantly reduced compared to when the car was new. This directly reflects that the actual energy released by the battery has decreased.
Increased internal resistance. You may find that it takes much longer to charge the battery to the second half (e.g. above 80%) than before. When performing operations that require instantaneous high current output, the device may also suddenly freeze, degrade in performance, or even shut down directly. This is because high internal resistance causes the voltage to drop sharply under high current.
Increased self-discharge rate. Healthy batteries will also self-discharge when not in use, but this rate may increase significantly as they age. If you find that a fully charged device, even when not in use, is losing power faster than when it was new, this is usually a sign of battery aging.
Battery bulging. This is a physical phenomenon that requires special vigilance. If the back cover of your phone is lifted up, or you can see that the battery area of the device has obvious expansion and deformation (bulging), this is usually caused by the decomposition of the electrolyte inside the battery to produce gas. This is a clear signal of internal failure and potential safety hazards, indicating that the battery structure may be unstable.
Practical tips for extending battery life
After understanding the internal and external factors of lithium battery aging, you can take some effective measures to maintain the battery and extend its effective service life as much as possible.
Temperature management is key to extending battery life. Avoid exposing devices with lithium batteries to high temperatures for long periods of time. Extreme cold also requires attention, especially during charging. If the device needs to be charged after being used in a cold environment, it is best to pre-charge with a small current first, then charge normally.
Optimize your charging habits. Your daily charging method has a subtle impact on battery life. Lithium batteries don’t like to be in a “full charge” or “low charge” state for a long time. Be sure to use chargers and data cables that are original to the device or have guaranteed quality and matching specifications. Use fast charging moderately, and don’t just pursue the convenience of fast charging.
Pay attention to the storage method. It is recommended not to store the device in a fully charged or fully discharged state. It is recommended to store the device in a cool and dry environment and avoid high temperatures.
Conclusion
The degradation of lithium batteries is a natural law of internal chemical and physical changes. By understanding the influence of factors such as temperature, charging habits, and usage intensity, we have mastered the initiative to delay degradation. Wise use and maintenance can indeed significantly extend battery life.
Resource
FAQs
Lithium ion batteries begin to degrade after their first use. However, the degradation rate of batteries is not entirely consistent, influenced by factors such as usage conditions, temperature, and charging habits. Normally, most lithium-ion batteries degrade to 80% of their initial capacity after 500 to 2000 cycles, which is also known as the battery's "cycle life".
Yes, even without use, lithium-ion batteries can still experience calendar aging. It is an irreversible loss caused by internal chemical reactions, independent of usage frequency. It is recommended to store the battery within the range of 30% -50% of its capacity and place it in a dry, cool environment to minimize the aging rate.
When battery performance declines, it manifests as a decrease in capacity and power. You may notice an increase in device charging frequency and charging time. At the same time, the battery may not be able to maintain its rated output under high loads, affecting the user experience of the device. In addition, as the internal resistance increases, the battery may generate more heat during operation, and even become hot to the touch.
High temperature environment: Choose high-temperature batteries, such as LiFePO4 (lithium iron phosphate) batteries with high heat resistance, and equip them with thermal management systems, such as air-cooled or liquid cooled systems.
High load application: Choose high rate power batteries to ensure stable operation of the battery even under high load conditions.
Optimize usage strategy: Control the charging and discharging depth of the battery (such as maintaining it within the range of 30% ~ 80%) to avoid deep discharge and overcharging.
